Blog
 Medical Device Project Management Operations are responsible for ensuring that unnecessary delays, and the resulting costs, are avoided. Optimizing the project plan, budget, and improving work efficiency – that’s the real-life story. Every new project should begin with a well-structured plan, something they stakeholders fully understand. People of PMO also make sure that all resources are allocated efficiently, preventing the waste of both team potential and materials. I decided to talk with Kamil Bobrowski – Consonance Head of PMO to find out where the drill is.
Medical Device Project Management Operations are responsible for ensuring that unnecessary delays, and the resulting costs, are avoided. Optimizing the project plan, budget, and improving work efficiency – that’s the real-life story. Every new project should begin with a well-structured plan, something they stakeholders fully understand. People of PMO also make sure that all resources are allocated efficiently, preventing the waste of both team potential and materials. I decided to talk with Kamil Bobrowski – Consonance Head of PMO to find out where the drill is.
 Neonatology startups aim to take care of newborns, especially premature babies. These highly vulnerable beings require exceptional care, as even the smallest changes can significantly impact their health and development. Sometimes, even the experience and knowledge of doctors aren’t enough to save the little one. Therefore, in a world where technology plays an increasingly vital role, neonatology startups become invaluable support for medical care.
Neonatology startups aim to take care of newborns, especially premature babies. These highly vulnerable beings require exceptional care, as even the smallest changes can significantly impact their health and development. Sometimes, even the experience and knowledge of doctors aren’t enough to save the little one. Therefore, in a world where technology plays an increasingly vital role, neonatology startups become invaluable support for medical care.
 3D bioprinting technology is an advanced integration of several engineering disciplines, including electronic engineering, tissue engineering, and bioengineering. It is based on the use of so-called bioinks, which consist of hydrogel, biomaterials, and patient cells. Layers of bioink are precisely applied in specialized printers, creating three-dimensional structures that can later be introduced into the body.
3D bioprinting technology is an advanced integration of several engineering disciplines, including electronic engineering, tissue engineering, and bioengineering. It is based on the use of so-called bioinks, which consist of hydrogel, biomaterials, and patient cells. Layers of bioink are precisely applied in specialized printers, creating three-dimensional structures that can later be introduced into the body.
 Isn’t a drug-device combination the best possible approach to join BioTech and MedTech innovations, despite their differences? They share a common goal: to support medicine in providing the best possible care and opportunity for speedy recovery. In most cases, a single approach to a disease is sufficient: either drug therapy or the use of medical devices. However, sometimes this is not enough for a complex problem, and a combined effort is required. The task theoretically seems simple, but in practice, it often proves to be more challenging.
Isn’t a drug-device combination the best possible approach to join BioTech and MedTech innovations, despite their differences? They share a common goal: to support medicine in providing the best possible care and opportunity for speedy recovery. In most cases, a single approach to a disease is sufficient: either drug therapy or the use of medical devices. However, sometimes this is not enough for a complex problem, and a combined effort is required. The task theoretically seems simple, but in practice, it often proves to be more challenging.
 It is often said that medical regulations are for a reason. There is no room for mistakes in the medical industry. What if a minor error could have dire consequences, and in extreme cases, it can cost human lives? Therefore, every medical device startup before releasing a product to the market, must face a series of requirements.
For most people (especially when you build your product for the first time), understanding the complexities of the law or keeping up with changes feel like navigating an endless maze. Non-compliance with regulations means that startups’ medical devices will not be allowed on the market, and they may also face fines. Fortunately, there are individuals who know how to navigate this maze and are capable of guiding companies through the complex regulatory processes.
It is often said that medical regulations are for a reason. There is no room for mistakes in the medical industry. What if a minor error could have dire consequences, and in extreme cases, it can cost human lives? Therefore, every medical device startup before releasing a product to the market, must face a series of requirements.
For most people (especially when you build your product for the first time), understanding the complexities of the law or keeping up with changes feel like navigating an endless maze. Non-compliance with regulations means that startups’ medical devices will not be allowed on the market, and they may also face fines. Fortunately, there are individuals who know how to navigate this maze and are capable of guiding companies through the complex regulatory processes.
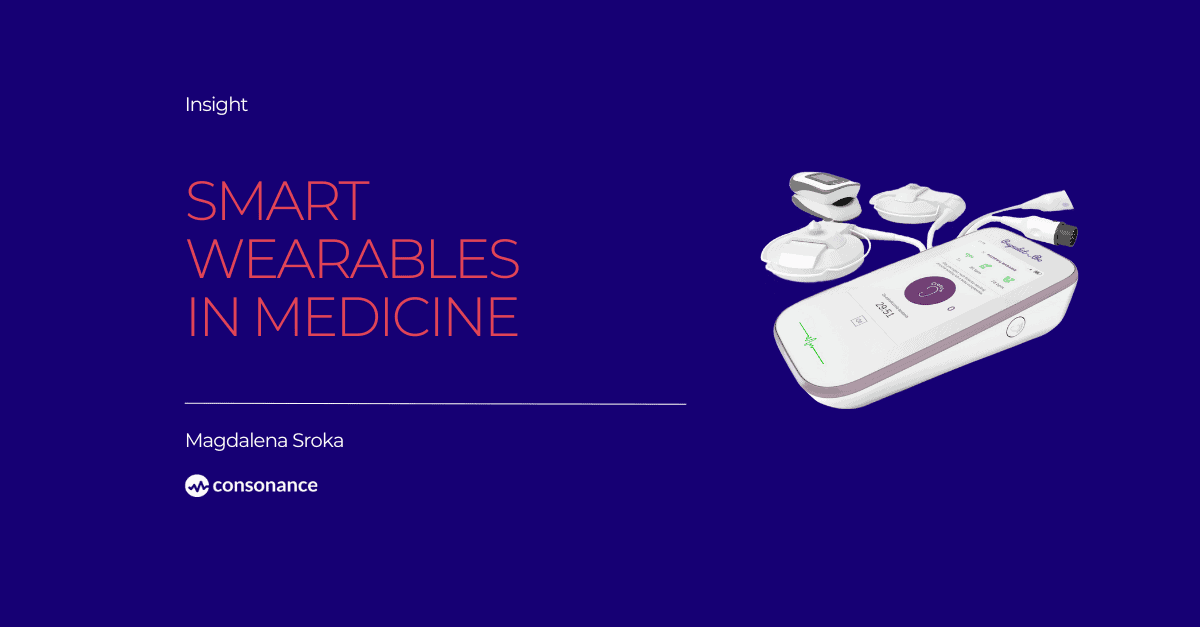 Most people associate smart wearables only with smartwatches or fitness trackers, but this is only a tip of an iceberg in possibilities where this technology can be used. These advanced electrical gadgets are designed to be worn on the user’s body. They are equipped with sensors and processors of various kinds which enables them to be connected with other devices as well as with the internet. It allows them to gather, analyze and exchange data in real time. In medicine it has found its use for monitoring health and condition, communication and for personal requirements.
Most people associate smart wearables only with smartwatches or fitness trackers, but this is only a tip of an iceberg in possibilities where this technology can be used. These advanced electrical gadgets are designed to be worn on the user’s body. They are equipped with sensors and processors of various kinds which enables them to be connected with other devices as well as with the internet. It allows them to gather, analyze and exchange data in real time. In medicine it has found its use for monitoring health and condition, communication and for personal requirements.
 Launching a medical device startup involves dealing with many variables influencing success. One critical factor is speed to market. Successful startups often move swiftly and gain momentum during commercialization. However, minor setbacks can slow development, significantly impacting time to market and financial outcomes.
Launching a medical device startup involves dealing with many variables influencing success. One critical factor is speed to market. Successful startups often move swiftly and gain momentum during commercialization. However, minor setbacks can slow development, significantly impacting time to market and financial outcomes.
 There’s a dynamic duo that’s been quietly saving the day: engineers and their knack for MedTech innovation. Medical technology has been turning heads with its life-saving devices and game-changing solutions. It’s the engineers who are pulling the strings, making magic happen from scratch. This article is all about shining a light on the engineers role in shaping the future of MedTech.
There’s a dynamic duo that’s been quietly saving the day: engineers and their knack for MedTech innovation. Medical technology has been turning heads with its life-saving devices and game-changing solutions. It’s the engineers who are pulling the strings, making magic happen from scratch. This article is all about shining a light on the engineers role in shaping the future of MedTech.
 Kickstart your medical startup: The 3rd edition of the Mother and Child Startup Challenge is here!
Are you a visionary entrepreneur with groundbreaking ideas in MedTech or Digital Health field? The Mother and Child Institute invites you to join the 3rd Edition of the Mother and Child Startup Challenge!
Kickstart your medical startup: The 3rd edition of the Mother and Child Startup Challenge is here!
Are you a visionary entrepreneur with groundbreaking ideas in MedTech or Digital Health field? The Mother and Child Institute invites you to join the 3rd Edition of the Mother and Child Startup Challenge!
 Can you imagine a pediatric healthcare today without MedTech solutions? Without a support of technology, both hardware and software? Why do they play a crucial role in disseminating and personalizing patient care, especially for the youngest ones? Children require a dedicated approach and specialized medical solutions that not only address their unique physical needs but also consider psychosocial aspects.
Can you imagine a pediatric healthcare today without MedTech solutions? Without a support of technology, both hardware and software? Why do they play a crucial role in disseminating and personalizing patient care, especially for the youngest ones? Children require a dedicated approach and specialized medical solutions that not only address their unique physical needs but also consider psychosocial aspects.
