e-Book: How to simplify medical device development?
Medical device development and commercialization process is commonly described as a complex subject. Various experts in the field use abstract terminology and ideas to explain the processes of design, prototyping, quality management and manufacturing. If you are a little like me, you may feel overwhelmed by terms like Quality Management System, Notified Body, Identification and Traceability, Nonconformities, MDR or Post-Market Surveillance, all in the first five minutes of most MedTech podcasts. If you are looking for clear, step-by-step instructions, you may find that existing resources can be vague and imprecise, as they aim to cover a wide range of scenarios. A different approach can be adopted: learning through example.
Table of Contents
Simplifying a medical device complexity
By following the development of other medical devices, you can uncover the right questions and gain a deeper understanding of the roadmap for medical device development.
The Consonance team prepared a demonstrational Technical Feasibility Study to provide an example on how to analyze the product requirements, how to consider their implementation and how to find the best pathway to make a device. We based the report on an ECG Holter device – a real-life example of a widely-used medical device.
Consider this Technical Feasibility Study as a starting point to evaluate whether your innovative idea is actually valid, feasible and what are the key components concerning its design and development.
Such a Study contains:
- Analysis of the intended use and functional requirements.
- State of knowledge analysis.
- Reference solutions analysis.
- Risk assessment.
- Analysis of the regulatory requirements.
- Technical solution concept.
A well-executed Technical Feasibility Study serves as a foundation for making informed design decisions, reducing development risks, overall costs and increasing the likelihood of regulatory approval.
While considering a new medical device we should specify its intended purpose and functionalities. Then, the in-depth state of knowledge analysis can provide an insight into our specific use scenario and the principle of work of the device. Based on the gathered knowledge the analysis of reference solutions can be performed. It can point to a direct reference of the designed device, highlight the issues with using the current solutions or find aspects influencing the development process.
The following section contains a part of reference products analysis with a shortened summary, to present just a gist of how the analyzes may look. The actual analysis of the reference solutions contains descriptions of 19 products and is preceded by functional requirements analysis and conclusions from the state of knowledge overview.
Reference solutions analysis in MedTech
ECG signal analysis is one of the most common diagnostic methods, providing good insights into the condition of the human heart. Holter monitoring is used for a screening and diagnosis of atrial fibrillation. According to the “Heart disease and stroke statistics—2019 update: a report from the American Heart Association” – the number of people hospitalized with atrial fibrillation is more than 454 000 each year and following CDC Wonder Online Database – around 158 000 deaths annually in the United States alone.
The chapter presents a partial overview of reference solutions available on the market that are comparable in terms of operation and purpose with the designed ECG Holter monitor device.
The following product groups were analyzed:
- cable monitors,
- patch monitors.
Cable monitors
End-users of medical devices benefit from an additional layer of identification. The Medical Device Regulation (MDR) imposes the Unique Device Identification (UDI) code system. While the UDI system is a comprehensive topic, it is a pivotal aspect of ensuring the identification and authenticity of medical devices in the market.
SEER 1000
SEER 1000 is a Holter device manufactured by Getemed for GE Healthcare. It allows recording of 2–3 ECG channels via 3-, 5- or 7-lead cables. The recording duration can be 24 h, 48 h or 7 days, depending on the device version. The SEER 1000 device is CE-certified and FDA-cleared.

SEER 1000
The device’s technical features are:
- ingress protection at IP43 level,
- sampling rate of 256 Hz for 12-bit resolution,
- a replaceable, single AAA battery,
- data transfer by USB 2.0 (with additional cable plugged to ECG connector),
- pacemaker impulse detection on all channels.
The device has a simple user interface with a single button allowing power on/off and marking of the patient’s event. It can be carried in a holder attached to a belt. The device has Bluetooth connectivity with a dedicated application available for Windows PC and iOS smartphones. The application allows the operator to input the patient’s data, configure the device, verify the signal quality and start the recording. The use of the application is optional. The recorded data can be analyzed and evaluated by a physician or experienced specialist. GE Healthcare provides the CardioDay 2.6 Holter ECG application for the signal analysis.
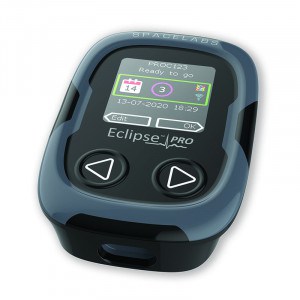
Eclipse Pro
The Eclipse Pro by Spacelabs Healthcare records a 3- or 12-channel ECG, depending on the chosen set of electrodes. The 3-channel recording can be done for up to 14 days and the 12-channel option is limited to 72 h. The device is CE-certified and FDA-cleared.
Eclipse Pro
The device’s technical features are:
- ingress protection rating at IP55 level,
- sampling rate of 8 kHz (signal is saved with lower sampling rate),
- rechargeable battery,
- data transfer by USB-C,
- pacemaker impulse detection.
The device is equipped with navigation buttons, a patient event button and a display for the initial signal quality check. The connector is adjustable for both standard electrode sets and a patch electrode. In the former case, the device is carried by a neck lanyard or belt clip. The device also has an additional functionality to detect lead-offs and alert patients with vibrations.
The accompanying software for the Eclipse Pro consists of:
- The Sentinel system.
- The Pathfinder SL Holter Analysis System.
- LifeScreen Pro personal computer application.
- Symptoms mobile application for the user.
PocketECG
PocketECG by Medicalgorithmics is a complete diagnostic solution for ECG recording. The monitoring duration of the device ranges from 24 hours up to 30 days. The PocketECG device and algorithm are CE-certified and FDA-cleared.

Pocket ECG
The device’s technical features are:
- ingress protection rating at IP20 level,
- sampling rate of 300 Hz,
- rechargeable battery,
- data transfer by 4G LTE or microSD card,
- pacemaker impulse detection.
The device is controlled via a touchscreen. The menu enables settings, signal preview and patient event marking. The patient’s events can be reported by selecting a symptom and an activity. Additionally, the patient’s physical activity is tracked by an accelerometer. The resolution of the ECG signal measurement was not specified by the manufacturer.
The PocketECG product consists of the recorder, user manual and PC client software. The device establishes consistent, remote ECG data flow from three leads and provides access to the ECG signal throughout the examination, along with a diagnostic report. The software enables the clinician to download and view the data from the remote server.
Patch monitors
Zio XT
Zio XT by the iRHYTHM is a wireless and continuous monitor that allows single-lead ECG signal recording for up to 14 days. The Zio XT device is CE-certified and FDA-cleared.

Zio XT
The device’s technical features are:
- ingress protection rating at IPX4 level,
- sampling rate of 200 Hz for 10-bit resolution,
- replaceable battery,
- no pacemaker impulse detection.
The device is prescription-only and for single-patient use. It has 2 electrodes and an event button for marking symptoms during the examination. The ECG recordings are stored internally within the device. The device is sent back to iRhythm after the recording, where heart data is analyzed for the patient’s doctor. The data are accessible at the end of monitoring in the final XT patient report, which is generated by an FDA-cleared deep learning algorithm.
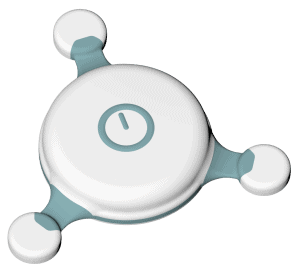
C3+ Holter Monitor
C3+ Holter Monitor by Cortrium records 3-channel ECG. The device stores recordings for up to 25 days and its battery capacity allows constant use for up to 7 days. The Cortrium C3+ device is CE-certified.
Cortrium C3
The device’s technical features are:
- ingress protection rating at IP54 level,
- sampling rate of 256 Hz for 24-bit resolution,
- rechargeable battery,
- data transfer by microUSB,
- no pacemaker impulse detection.
The device can be used with standard ECG electrodes. The measurement is started by a medical professional in a clinic. The C3+ device is controlled with one button. A single press turns on the device and marks a patient’s event. The state of the device is signalized by an LED light. Additionally, for patient setup and data upload, the Cortrium Apex cloud-based software is available. The reports are generated after software analysis and human verification.
Walk Free
Walk Free from Cardioline is a patch recording a 3-channel ECG manufactured by livetec (variants used by other companies are also available on the market). The duration of the examination is up to 9 days. The Walk Free device is CE-certified.

Cardioline Walk Free
The device’s technical features are:
- ingress protection rating at IP54B level,
- sampling rate of 250 Hz for 16-bit resolution,
- a replaceable AAA battery,
- data transfer by USB 2.0,
- no pacemaker impulse detection.
Walk Free is a wireless recorder measuring leads I, II and III. The placement of three standard electrodes is directly on the patient’s chest. The measurement begins automatically after starting the software or inserting the battery with a 5-minute delay.
The ECGWebApp Holter of Cardioline is a web-based platform managing the complete Holter workflow, encompassing reporting, storage and examination of Holter ECG studies. The software can be used with Holter devices by Cardioline and other devices from different manufacturers.
Conclusions
The main conclusions of the reference solutions analysis, concerning the device development are:
- A substantial number of devices available on the market presents a variety of technical and functional aspects. Most of the analyzed solutions can be considered as direct equivalents to the designed product.
- The designed device should be treated as an integral part of the systemic solution, combined with applications and analysis software.
- The high sampling rate or the increased number of channels do not stand out from the large number of current solutions. High technical performance does not seem to be the most important aspect of Holter devices.
- Taking into account the high diversity of reference solutions and the automation of the recording and diagnostic processes, it seems that usability is a crucial aspect of the Holter monitoring device’s design. It is worth considering additional functionalities of the device, such as electrode disconnection detection or connection quality assessment, to support the setup process.
Get the full report for free
The report was prepared by Consonance – a one-stop medical device company focused on prototyping and engineering active medical devices, with a proven track record of 50+ projects delivered.
Inside the full report you’ll find the prioritization of the functional requirements, a deep dive into measurement procedures and errors, an extensive analysis of the reference products, the regulatory classification of the designed device, identification and analysis of the applicable standards, the concept of technical solution from scratch and conclusions highlighting the most crucial aspects of the project.







