Early Medical Device Prototyping: A Strategic Investment for MedTech Startups
Medical device prototyping is the inherent piece of the development process. There’s no shortcuts. This means new medical devices can cost thousands or millions of Euros / Dollars to develop, largely due to repeated design iterations and heavy regulatory testing. Another factor is the device’s class. The higher class you aim for, the more sophisticated product you want to achieve.
With this complexity comes risk, so you need to spend more time on a wise and targeted prototyping process. Crucially, “iterative prototyping” itself is a major cost driver – meaning smarter prototyping can cut total expenses by 20 – 30%. In practice, building quick prototypes early uncovers design flaws before expensive tooling, refines usability, and confirms manufacturability. Rapid prototyping significantly reduces the time and cost associated with early-stage development. In short, every dollar you spend on smart prototyping can save many more down the road by avoiding costly rework or compliance delays.
This longevity requirement influences component selection, manufacturing processes, and software updates. Unlike smartphones, where minor software bugs can be patched later, medical device software must be nearly flawless from the outset. Even small software updates often require re-certification, adding another layer of complexity to product development.
Importantly, the team used these prototypes not only for technical iteration, but also for usability testing with clinicians, ensuring the final design would integrate seamlessly into routine care. Because Consonance’s team embedded manufacturing constraints and CE certification planning into the prototype stage, they avoided late-stage redesigns or certification delays. The result was a clinically reliable, user-centered diagnostic tool that could be scaled at a reasonable cost. This strategic prototyping process reduced development risk and paved the way for efficient production of a complex yet cost-sensitive diagnostic device.
Additionally, Consonance worked to align the electronics and form factor with scalable production capabilities early on, which avoided late-stage redesigns that often increased costs. As a result, the final device offers clinical-grade performance and is optimized for mass production without compromising patient comfort or safety.
At the same time, the engineers integrated manufacturing constraints – such as component availability and housing mold complexity – into the design process, ensuring scalability from the very first iteration. This foresight reduced future redesign efforts, lowered tooling costs, and ensured that the final product would be efficient to produce while maintaining high clinical standards.
Several iterations focused on refining dry electrode technology and developing a comfortable yet stable headset that non-specialist personnel could easily use. This disciplined, cost-conscious approach enabled BrainCapture to create a reliable and scalable medical device tailored for global health markets – proving that affordability and quality can coexist when prototyping is handled strategically.
The final product features a three-step professional treatment program with 30 intensity levels, delivering non-invasive pain relief for conditions like back pain, sciatica, and muscle tension. By integrating manufacturing considerations early in the prototyping process, Consonance ensured that TRU Plus could be produced cost-effectively at scale, making advanced pain therapy accessible to a broader patient population.
The bottom line for founders: invest in early prototyping to de-risk your project. It may feel like an extra up-front cost, but in MedTech the savings pay for themselves. As one analysis puts it, engaging regulators early and using lean methods can trim over half of development costs. Rapid prototyping empowers you to catch issues while they’re cheap to fix, refine your design for efficient manufacturing, and ultimately offer a better-priced product. By following the lead of the above startups – and even designing prototypes to mirror final materials and processes – your team can accelerate the go-to-market timeline and build in competitive pricing from day one. In short, thoughtful prototyping isn’t a luxury for MedTech startups; it’s a cost-management strategy that drives long-term profitability.
If you’re here we kindly invite you to learn more about our experience taken from developed & commercialized medical products for our clients. Let’s move yours ahead of the curve.
Contact us now!
With this complexity comes risk, so you need to spend more time on a wise and targeted prototyping process. Crucially, “iterative prototyping” itself is a major cost driver – meaning smarter prototyping can cut total expenses by 20 – 30%. In practice, building quick prototypes early uncovers design flaws before expensive tooling, refines usability, and confirms manufacturability. Rapid prototyping significantly reduces the time and cost associated with early-stage development. In short, every dollar you spend on smart prototyping can save many more down the road by avoiding costly rework or compliance delays.
Early Medical Device Prototyping Benefits
- Catching design errors before tooling: Functional prototypes (3D-printed or machined models) reveal flaws in form, fit or function before you invest in mass-production molds. This saves wasted tooling costs. Optimizing prototyping can cut development costs by ~20–30%, but this also depends on the device’s type.
- Design for Manufacturability (DFM): By trying out assembly and materials early, teams ensure the design can scale. For example, redesigning a pump’s plastic housing for two-shot molding (instead of hand-soldered electronics) can slash per-unit build time and cost. Think about this: “Manufacturability from the start!” and keep it as your development motto.
- Regulatory vetting: Early prototypes enable usability testing and pre-validation under ISO/FDA standards. Consonance’s projects, for instance, embed ISO 13485/CE certification planning into prototyping from day one, catching compliance issues before they derail progress.
- Faster iteration and feedback: When you can build and test dozens of variations quickly (e.g. via 3D printing or soft molds), you learn what works before committing to production. This lean approach trims months off timelines.
- Better unit-cost/pricing: Ultimately, a refined design means lower manufacturing cost per part, which translates to healthier margins or more competitive pricing. (We’ll see examples below where prototyping choices cut per-unit costs from hundreds to single digits.
MedTech Startup Case Studies: Prototyping in Action
Consumer electronics are designed for frequent updates and short product cycles-new smartphone models emerge every year. Medical electronics, however, must remain stable and functional for long periods, sometimes decades, with minimal updates. A pacemaker, for example, must last for years inside the human body without malfunctioning.This longevity requirement influences component selection, manufacturing processes, and software updates. Unlike smartphones, where minor software bugs can be patched later, medical device software must be nearly flawless from the outset. Even small software updates often require re-certification, adding another layer of complexity to product development.
ONIRY Postpartum Diagnostic Device
Developed in partnership with Consonance, ONIRY is a diagnostic device that uses impedance spectrometry to monitor pelvic floor recovery and detect postpartum complications. From the outset, prototyping was approached with a focus on medical accuracy, manufacturability, and user comfort. Early functional prototypes allowed engineers to test electrode placement, validate signal stability, and refine the device’s form factor for postnatal use.Importantly, the team used these prototypes not only for technical iteration, but also for usability testing with clinicians, ensuring the final design would integrate seamlessly into routine care. Because Consonance’s team embedded manufacturing constraints and CE certification planning into the prototype stage, they avoided late-stage redesigns or certification delays. The result was a clinically reliable, user-centered diagnostic tool that could be scaled at a reasonable cost. This strategic prototyping process reduced development risk and paved the way for efficient production of a complex yet cost-sensitive diagnostic device.
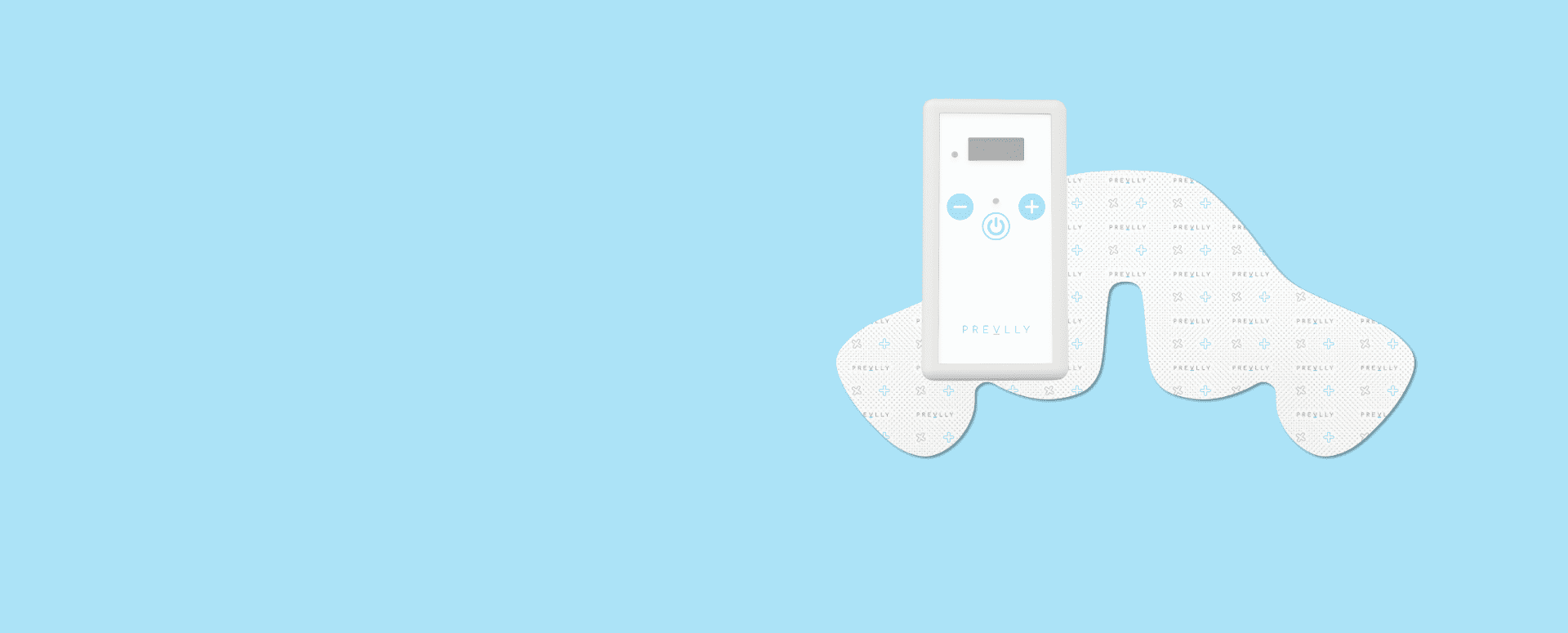
Prevlly – Preventive Pressure Ulcer Patch
Prevlly is a next-generation wearable patch designed to prevent pressure ulcers through controlled electrostimulation. During prototyping, Consonance’s team conducted a technical feasibility assessment that helped define key parameters such as electrical stimulation safety, skin contact reliability, and power consumption. Iterative prototyping revealed several usability concerns – such as skin irritation, adhesion time, and battery life – which were solved before moving to clinical validation.Additionally, Consonance worked to align the electronics and form factor with scalable production capabilities early on, which avoided late-stage redesigns that often increased costs. As a result, the final device offers clinical-grade performance and is optimized for mass production without compromising patient comfort or safety.
Pregnabit Pro – Telemedical CTG Device
Pregnabit Pro is a compact telemedical cardiotocography (CTG) system designed for use both in hospitals and at home. The prototyping process focused heavily on user-centered design, especially the ergonomics of wearable sensors and wireless data transmission. Consonance’s team developed and tested multiple prototypes to validate fetal heart rate signal accuracy and maternal comfort, which led to significant improvements in device placement and UI/UX.At the same time, the engineers integrated manufacturing constraints – such as component availability and housing mold complexity – into the design process, ensuring scalability from the very first iteration. This foresight reduced future redesign efforts, lowered tooling costs, and ensured that the final product would be efficient to produce while maintaining high clinical standards.
BrainCapture – Low-Cost Mobile EEG for Epilepsy Detection
BrainCapture set out to revolutionize epilepsy diagnosis with a mobile EEG device that could be deployed in low-resource settings. Consonance supported this vision by engineering a prototype optimized for ultra-low cost without compromising signal quality. During the prototyping phase, careful attention was paid to minimizing the number of components, simplifying the electronics, and choosing durable, off-the-shelf materials to keep the bill of materials lean.Several iterations focused on refining dry electrode technology and developing a comfortable yet stable headset that non-specialist personnel could easily use. This disciplined, cost-conscious approach enabled BrainCapture to create a reliable and scalable medical device tailored for global health markets – proving that affordability and quality can coexist when prototyping is handled strategically.
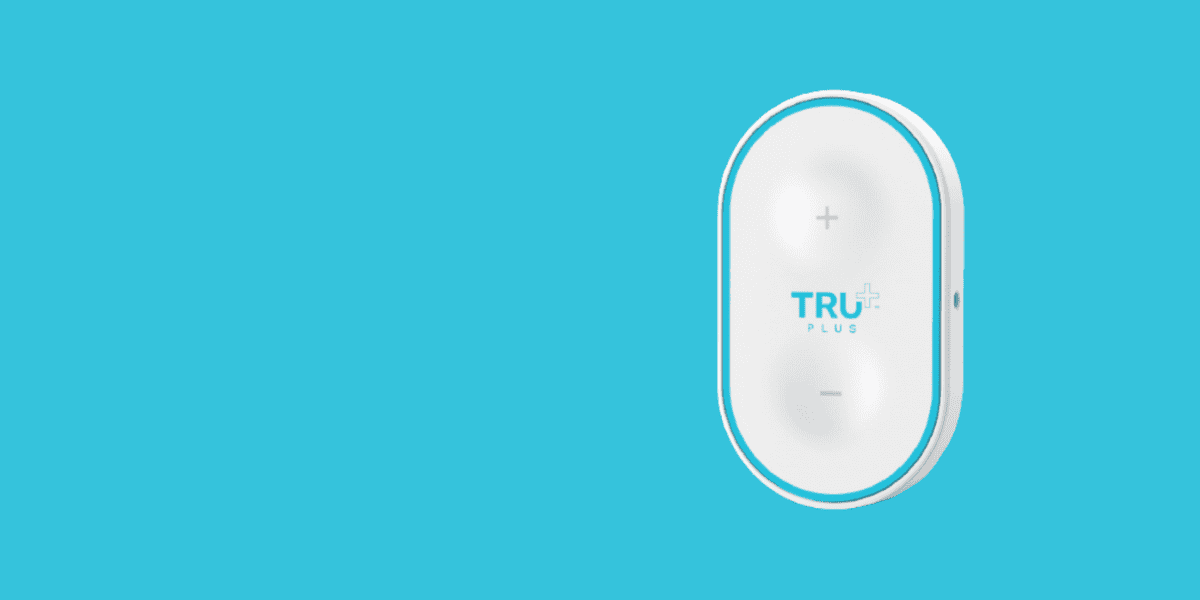
TRU Plus – Portable TENS Device for Pain Relief
TRU Plus is a portable transcutaneous electrical nerve stimulation (TENS) device developed by Consonance for USP Zdrowie, one of Poland’s largest pharmaceutical companies. The project spanned three years, encompassing design, prototyping, testing, ISO 13485 implementation, and preparation for serial production. The prototyping phase was crucial in refining the device’s ergonomics, user interface, and electrical stimulation parameters to ensure both efficacy and user comfort.The final product features a three-step professional treatment program with 30 intensity levels, delivering non-invasive pain relief for conditions like back pain, sciatica, and muscle tension. By integrating manufacturing considerations early in the prototyping process, Consonance ensured that TRU Plus could be produced cost-effectively at scale, making advanced pain therapy accessible to a broader patient population.
Early Medical Device Prototyping Benefits
These examples from Consonance make it clear: prototyping decisions shape manufacturing and pricing. Strategic prototyping can lead to substantial cost savings, regulatory readiness, and smoother manufacturing transitions. It allows MedTech entrepreneurs to validate assumptions early, build confidence with stakeholders, and streamline the path to market. More than a technical checkpoint, prototyping is a business-critical phase that directly influences your device’s success – and its affordability.The bottom line for founders: invest in early prototyping to de-risk your project. It may feel like an extra up-front cost, but in MedTech the savings pay for themselves. As one analysis puts it, engaging regulators early and using lean methods can trim over half of development costs. Rapid prototyping empowers you to catch issues while they’re cheap to fix, refine your design for efficient manufacturing, and ultimately offer a better-priced product. By following the lead of the above startups – and even designing prototypes to mirror final materials and processes – your team can accelerate the go-to-market timeline and build in competitive pricing from day one. In short, thoughtful prototyping isn’t a luxury for MedTech startups; it’s a cost-management strategy that drives long-term profitability.
If you’re here we kindly invite you to learn more about our experience taken from developed & commercialized medical products for our clients. Let’s move yours ahead of the curve.
Contact us now!