Medical device manufacturing
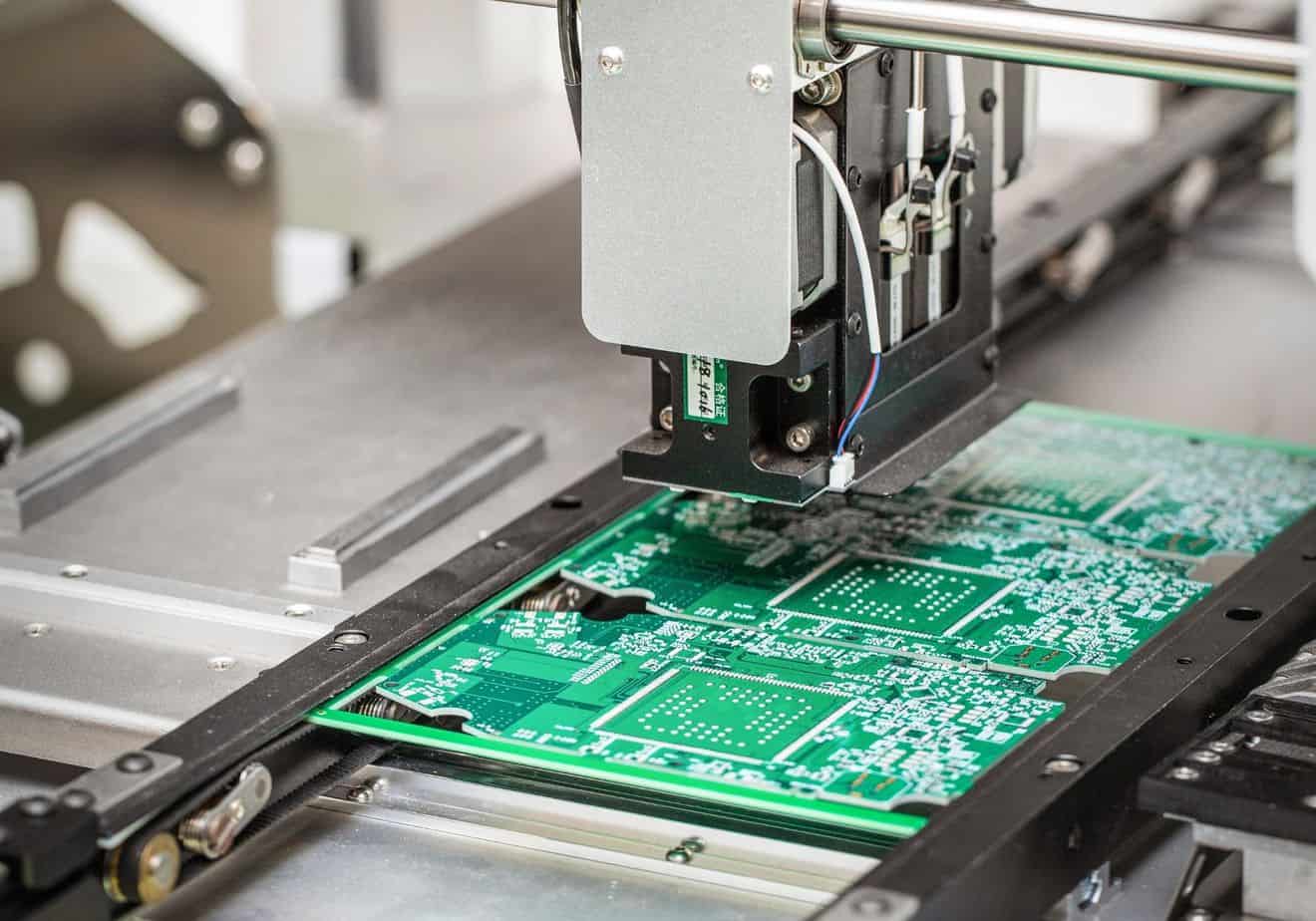
Table of Contents
ISO 13485-certified medical device manufacturing company
Consonance is an ISO 13485-certified medical device manufacturing company. We can assist you in your project by managing the entire production process of your device, from component and supplier selection to design for manufacturing and the actual production of your device.
Our in-house production capabilities include:
- mass production of up to 5,000 device units per month (final assembly, testing, quality control, packaging)
- production of trial series
- development and production of functional prototypes and production prototypes of medical devices
- supply chain management
- material and component selection and sourcing
- selection, auditing and management of suppliers and subcontractors for specialised components.
Production of medical devices
We have a track record in the production of medical devices of classes I, IIa and IIb. We have an in-house manufacturing facility equipped with a 3D printer, a CNC machine, a solder paste printer, a pick & place machine, a reflow soldering oven, automated test equipment, a foil wrapping machine, and other production devices.
Contact us!
If you would like to learn more about how our medical device manufacturing services can get you ahead of the competition, just drop us a line or use the form below.



