MEDTECH INNOVATIONS
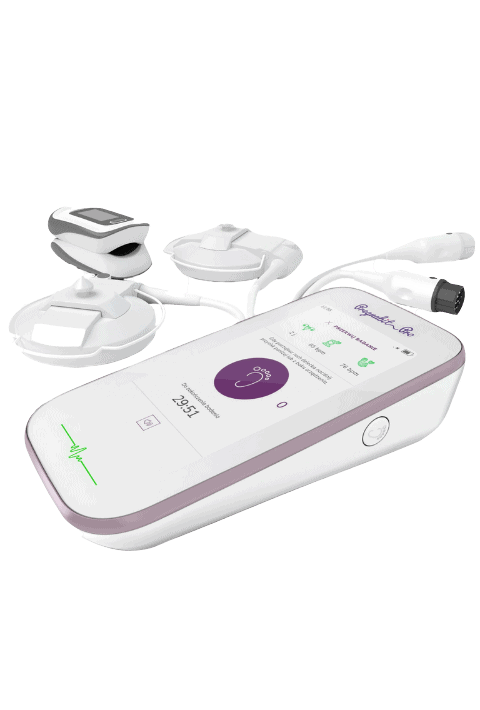
Medical device development services for MedTech startups
One-stop: everything startups need to successfully start, build, and grow a medical product.
We engineered 50+ medical startups to get them to the market.
From guidance in one stage or throughout the process from concept, design, hardware development to commercialization and MDR readiness.
That makes us special. We understand how startups strive. We offer direct assistance to bring high quality medical devices to market faster.
What clients say about us?

 Since June 2022, we have been working with Consonance throughout the development process up to and including the BC-1 medical devices manufacturing. Their knowledge in the technical and regulatory field has been a huge gain. The agreed deliveries: time, quality and price have been delivered without deviation. We expect to continue our cooperation.
Since June 2022, we have been working with Consonance throughout the development process up to and including the BC-1 medical devices manufacturing. Their knowledge in the technical and regulatory field has been a huge gain. The agreed deliveries: time, quality and price have been delivered without deviation. We expect to continue our cooperation.
Lisbeth Moller Christensen
QA Manager
BrainCapture
Denmark
QA Manager
BrainCapture
Denmark
 “We worked together with Consonance to prepare functional prototypes of our impedance spectrometry and anal probe, the main parts of the ONIRY product. The cooperation was very good, the project was perfectly managed and communicated, all work went according to the schedule. We are fully satisfied.”
“We worked together with Consonance to prepare functional prototypes of our impedance spectrometry and anal probe, the main parts of the ONIRY product. The cooperation was very good, the project was perfectly managed and communicated, all work went according to the schedule. We are fully satisfied.”
Katarzyna Borycka
PhD, CEO
OASIS Diagnostics
Poland
PhD, CEO
OASIS Diagnostics
Poland
 "Consonance exhibited a high level of expertise, demonstrated exceptional organizational skills and a highly structured approach in conducting the feasibility study. The result they provided was not only thorough but also actionable, laying a solid foundation for the subsequent stages of the prototype development."
"Consonance exhibited a high level of expertise, demonstrated exceptional organizational skills and a highly structured approach in conducting the feasibility study. The result they provided was not only thorough but also actionable, laying a solid foundation for the subsequent stages of the prototype development."
Melba Munoz
MD, PhD
Charite
Germany
MD, PhD
Charite
Germany
Our Clients
Helping clients with
Innovation on-time and on-budget
Launching a startup in the medical device industry is an exciting endeavor. Yet there are significant considerations, processes, and protocols for entrance.
For 9+ years we have been catalyzing medical device innovation by the likelihood of a successful commercialization by removing the complexities, cost barriers, complex medical regulations and time sensitivities related to introducing new medical products into the market.
We help to move over product development critical phases in order to establish your business as a sustainable competitor. We are ISO 13485 company and offer MDR certification support.
Let's talk about your next medical innovation!